Background
Febrile neutropenia (FN) is a potentially life-threatening complication of myelotoxic chemotherapy, with increased incidence in patients with high risk chemotherapy or with patients with predisposing comorbidities in intermediate risk regimens. Prophylactic pegfilgrastim has been shown to decrease the incidence, duration, and severity of neutropenia, fever, and infection. Current guidelines recommend at least a 24-hour time lapse from chemotherapy to administration of growth factor; however, many cancer centers give same day growth factor due to convivence. Here, we explored lymphoma patients treated with CHOP+/-R (cyclophosphamide, doxorubicin, vincristine, prednisone, with or without rituximab) and received either same-day (within 24 hours of chemotherapy) or next-day (≥24 hours post chemotherapy) pegfilgrastim to address the safety and efficacy of administration timing in the real-world setting.
Methods
A retrospective, single center, cohort study was conducted between October 1, 2013 and October 1, 2016 to evaluate lymphoma patients who were treated with CHOP+/-R and given pegfilgrastim for prophylaxis at the University of Arizona Cancer Center. Patients were followed up to six cycles of chemotherapy. In the first cycle, 39 patients were identified: 33 patients received same-day pegfilgrastim and 6 patients received next-day pegfilgrastim. Primary data collected included the incidence of FN, neutropenia, dose reductions, antibiotic administration, and hospitalization rate.
Results
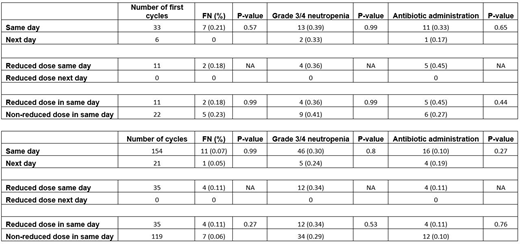
Two hundred and fourteen R-CHOP (0.90) or CHOP (0.10) chemotherapy cycles (187 cycles for the same-day and 27 cycles for the next-day) were evaluated in 39 patients. Among the first cycles (33 patients in the same-day and 6 patients in the next-day), there were no significant differences in the incidence of FN (0.21 vs. 0.00, P= 0.57), grade 3/4 neutropenia (0.39 vs. 0.33, P= 0.99) and hospitalizations (0.39 vs. 0.00, P= 0.08) between the same day and the next day groups. Among cycle two to six (154 cycles for the same-day and 21 for the next-day), there were no statistically significant differences in the incidence of FN (0.07 vs 0.05, P=0.99)grade 3/4 neutropenia (0.30 vs. 0.24, P= 0.80) and hospitalizations (0.11 vs 0.10, P= 0.99) between the same-day and next-day. The same-day group, had 33% percent of patients received reduced doses of CHOP or R-CHOP (median age= 80.0 yr). The incidence of FN was 0.11 in these patients and 0.06 in patients who received the usual doses (median age=65.5 yr) (P= 0.27). The incidence of grade 3/4 neutropenia was (0.34 vs 0.29, P=0.53) and hospitalizations (0.20 and 0.08, P=0.07) between patients who received reduced doses of chemotherapy because of age versus normal dosing respectively in the same-day arm.
Conclusion
In our analysis, we have shown that same-day was as safe and effective as next-day pegfilgrastim administration in lymphoma patients receiving CHOP+/-R. There was not a significant increase in FN in either group. Future prospective studies are warranted to investigate the practical benefits of same-day pegfilgrastim or its biosimilar. Potential future study directions could include addressing outcomes, side effects, patient satisfaction, and reduced healthcare costs associated with same-day administration of pegfilgrastim for the prophylaxis of FN.
Abraham:Coherus BioSciences: Research Funding, Speakers Bureau; Celgene: Consultancy; Terumo: Consultancy; Rockwell Medical: Consultancy; Janssen: Consultancy; Mylan: Consultancy; Sandoz: Consultancy; MorphoSys: Consultancy. McBride:Coherus BioSciences: Consultancy, Speakers Bureau; Merck: Speakers Bureau; Pfizer: Consultancy; Sandoz: Consultancy; MorphoSys: Consultancy; Bristol-Myers Squibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal